Background: Treatment of acute lymphoblastic leukemia (ALL) in adults has historically shown unsatisfactory outcomes when compared to the outstanding results obtained in pediatric patients. Pediatric protocols rely on increased drug intensity and on a tighter evaluation of minimal residual disease (MRD) for early intensification. Nowadays, pediatric-inspired protocols have been established as the standard of care for adolescents and young adult patients (AYA) leading to improved results. In the setting of older patients, probably due to the limited data available, the feasibility of such intensive therapies, and in particular the administration of high doses PEG-asparaginase (PEGASP), is still a matter of debate. In this context, the NOPHO group reported that a full-pediatric protocol was feasible and highly effective in adult patients, up to 45 years of age.
Aim: The aim of our study was to evaluate the feasibility and efficacy of the entire full pediatric AIEOP-BFM LAL 2009 protocol in an older cohort of adult patients up to 55 years.
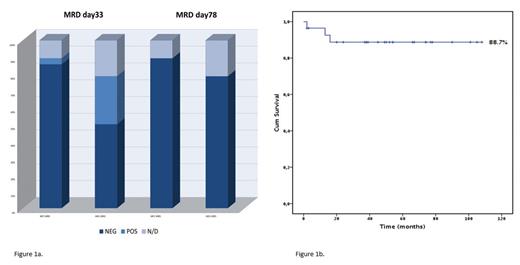
Methods: Since May 2013, 28 consecutive adults diagnosed with Ph-neg ALL received first line therapy according to AIEOP- BFM-ALL 2009 protocol, which includes high PEG-asparaginase (PEGASP) doses, with an accurate MRD-driven therapeutic strategy. The protocol consists of 2 induction blocks: IA (Vincristine, Daunorubicin, PEGASP, IT methotrexate) and IB (Cytarabine, 6-mercaptopurine, cyclophosphamide, IT methotrexate), I consolidation block (high-dose methotrexate, 6-mercaptopurine, IT methotrexate), maintenance therapy (6-mercaptopurine, weekly MTX, IT Methotrexate). Median age in our study was 45 years (range 17-55), equal to the maximum age allowed in the NOPHO study. Fifteen patients had B-ALL, 13 T-ALL/T including 5 Early-T phenotype. Dose intensification and stem cell transplantation (SCT) were scheduled according to baseline and MRD-driven risk assessment. MRD was evaluated by multicolor-flow cytometry (MFC), with a threshold for positivity of 0.025%, and molecular PCR for JH rearrangement (MOL), with a sensitivity greater or equal to 10-4 in all patients. Patients with MOL MRD >10-4 but <10-3 were defined as low-level positive. MRD time points were day 33 and day 78 (MFC and MOL).
Results: All patients achieved complete hematological remission following the first Induction course (100%). One patient died during induction due to infection by multidrug resistant P. aeruginosa. In general, therapy was well tolerated. Remarkably, patients in this study received high dose of PEGASP (median 10000 UI/sqm, equal to 4 administrations at 2500 UI/sqm each) without experimenting life threatening toxicities, which mostly consisted in asymptomatic elevation of transaminase (7 patients G3, 1 G4) and/or bilirubin (9 patients G3, 1 G4) only requiring supportive therapy. No major bleeding or thrombotic events o other >G2 toxicities were observed. All except one patient achieved CR at day 33 and all patients achieved CR at day 78. MFC MRD evaluation at day 33 resulted negative in 24/25 evaluable patients (95%). Day 33 MOL-MRD was evaluated in 22 patients, and resulted negative, low level positive or positive in 14, 5 and 3 patients, respectively. MRD assessment at day 78 showed both MFC and MOL negativity achieved in all 25 and 22 evaluated patients, respectively (Fig. 1). Four very high-risk patients underwent SCT in negative MRD status. After a median follow-up of 59 months (CI 95% 38.45-78.63), median survival was not reached, 5-years OS was 88.7% (Fig. 2). One patient died due to progressive transverse myelitis after isolated CNS relapse and one patient died due to sudden cardiac arrest while in complete MRD negative remission, 6 months after completion of therapy. All other 25 patients are alive and in complete MRD negative remission.
Conclusion: The application of a full pediatric induction regimen in a cohort of adult ALL patients with a median age of 45 years proved to be feasible, with tolerable toxicities. In particular, we didn't observe major toxicities during the intensified PEGASP administration and all patients were able to receive the planned PEGASP doses. The possibility to maintain the correct timing translated in a very high MRD negativity rate and promising OS survival.
Disclosures
No relevant conflicts of interest to declare.